WHITE PAPER
Presented by:
Marshall Manufacturing Company
Minneapolis, Minnesota
USA
Since the beginning of modern civilization, it seems man has been attempting to figure ways to bend various elements in order to perform specific tasks. The challenges along the way have often centered upon understanding how the tool needs to be constructed in order to perform the intended task with the least amount of failure-risk. These challenges exist today especially when considering the very intricate and specific world of medical devices. This study is intended to open awareness about how metal wire and tubing can be formed to exact specifications and what to consider when working with companies who claim to be experts in bending for the medical device market.
A myriad of variables concerning bent metal can be exhaustive to understand in a complete manner. Marshall Manufacturing has built a surplus of knowledge. Garnering expertise and claiming higher ranking within said knowledge can not be accrued through the written page. From their first attempt to bend stainless steel wire to specification, then adding machined features, they found the bend is likely to distort because of added stresses put into the material in the bending process. That’s when the real knowledge began. Many years ago, Marshall set itself on a path unaware.
At that time, not a soul within the company would have thought that Marshall Manufacturing would become the premiere metal wire and tube bending manufacturer preferred by medical device OEM’s around the globe. So, this is a paper about knowledge accrued; about bending wisdom to attempt control and ultimately offer customers a better way, a successful outcome. They call it “Cost Optimization Strategy”. The medical device OEM’s often call it “amazing”.
Design Intent, Feasibility and Definition.
Each phase has a specific outcome, must be done in a specific order, and cannot be skipped or short changed without impacting the overall cost of the project.
Design Intent
Let’s start at the beginning by explaining how Marshall defines Intent.
How is the part to be used? Where does it fit into the possible assembly? How will it function if in the surgeon’s hands? Without this information, the design cannot move forward. Design Intent starts with communication. What information is currently known about the device? It will most likely begin with the initially proposed print. The intent needs to be clear, understandable and cohesive. It is within this step of the process where the actual design begins to take shape. The goal of this phase is to reach consensus regarding Design Intent. What will the device do? What are the constraints? What are the limitations? All of which must be clarified, understood, dealt with and agreed-upon before entering the second phase of the medical device bending process.
A medical device can be described as a set of features that perform a given set of functions. During the Design Intent phase, a thorough understanding of these features and their interactions is imperative to developing an effective device.
Potential questions to ask during the Initial Design Review Phase (Intent):
- Are there opportunities to change/adjust the design of this component?
- Is this a component that must ‘mate’ with other components? Are there opportunities to change/adjust the design of mating components?
- Is there an ‘envelope’ that we need to work within?
- Will the components be reusable or disposable?
- What are the ‘must haves’? What are the critical characteristics of the design? What information can be provided regarding the design outputs?
- What information currently exists regarding the limits of acceptability for the critical characteristics?
- What is known regarding material requirements? Are there material types, tensile requirements, weight restrictions, environmental factors, or other variables that constrain options?
- What is known regarding the orientation / location requirements for the critical features?
- Are there specifications or standards that must be met?
- What are the finish requirements? Surface finish, burrs, heat treatment, plating, electropolishing, passivation, sterilization, cosmetics, etc. all need to be addressed/considered.
- What are the potential quantities that will need to be made (i.e. estimated annual usage)?
Often an initial draft of a bent part looks similar to Figure 1 (below).
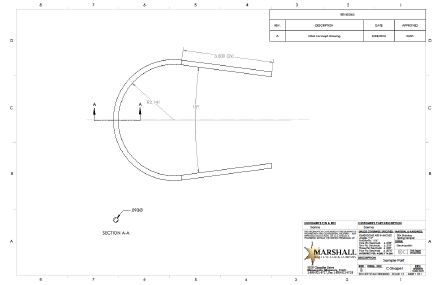
This drawing fully defines all nominal geometry, but doesn’t as clearly demonstrate design intent. Additionally, it’s drawn in a way that makes it extremely difficult to manufacture and even inspect.
If, for example, the part will be constrained by mating components and will be ‘pushed into place’ during assembly, the tight tolerances described by the title block may not be required. Perhaps the straight sections need to be held in space tightly in relation to each other, but the curve has a wide envelope of tolerance allowable. This type of information is vital to find the optimum design.
Additionally, each dimension of the geometry chosen in Figure 1 is toleranced individually. Often this is done because it takes less time than a more fully developed description of requirements. This choice often leads to increased costs down the road. Stumbling blocks during the Feasibility stage can be avoided by understanding Design Intent.
Feasibility
The purpose of the Feasibility phase of the design is to confirm that the requirements for the Design Intent can be met by the processes developed. Often manufacturers will enter the Feasibility phase first-only to find the part they are attempting to bend simply cannot perform to initial specifications. Within the Feasibility phase, medical device design engineers should know that the part can be made.
Full description of the Design Intent allows us to narrow down our choices for how to manufacture a part. Some processes are less expensive, but exhibit more variation, other processes narrow the disparity, but come with increased cost. Spending the effort to fully define Design Intent allows us to make the right choices regarding processing.
Outcomes from the Feasibility phase include:
- Confirmation that the performance of our manufacturing capability exceeds the requirements of the design. This includes confirmation that critical characteristics can be achieved (including tolerance requirements).
- Material requirements are defined and understood (including tensile requirements and other characteristics).
- Definition of machines / processes to be used.
- Description of the tooling requirements.
- Clarification of inspection processes.
- Processing costs, including labor, material, and equipment requirements.
Typically this phase is performed by making parts that meet the design requirements. Ideally, we use machines, tools, and materials that mimic those intended to be used for production quantities. This approach avoids the problems often seen when alternate methods are used for prototypes. Unforeseen production problems develop that then cause delays and additional costs, including the potential for revisiting the design.
In the early stages of a design, it’s common to not have fully defined limits for critical characteristics. Often the tendency is to tighten the tolerance by a significant amount to ensure that the part will function. This is often the more costly approach. The purpose of the Feasibility stage is to define limits of the design requirements based on specific acceptance criteria, instead of a ‘belt and suspenders’ approach holding overly tight tolerances or tolerances that don’t directly coincide with design requirements.
Another commonly overlooked problem occurs when the print calls out characteristics that are extremely difficult to check. In Figure 1 above, the 3.500” straight section blends into the 2.141” arc in a smooth continuous shape. This is easy to draw, but difficult to measure. Care must be taken regarding how transition points at tangents (either straight to curve or curve to curve) are called out.
Inspection requirements should ensure critical characteristics are met. This technique often results in a print modification that clarifies design requirements.
Upon completion of the Feasibility stage, part Definition can be finalized.
Definition
Once critical requirements are understood and feasibility of processes has been achieved, we can finalize the design in a coherent manner. In the world of bending metal wire and tubing, Geometric Dimensioning and Tolerancing (GD&T) is a powerful tool. True medical device engineers know that datum targets can be used in harmony to assist in defining specific requirements.
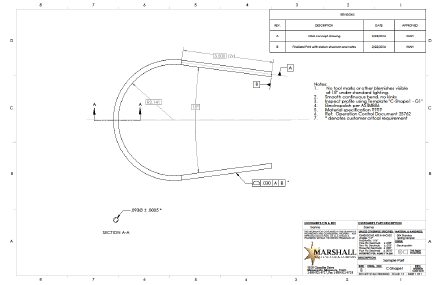
Figure 2 shows the finalized print. Note the following changes:
- Profile tolerance with datum structure calls out true bend requirements
- Customer critical dimensions are now noted
- Specifications for material and electropolish are noted
- Visual and cosmetic requirements have been added
- Operational control and inspection instructions have been noted (optional, but often useful).
Whereas Marshall Manufacturing has gained perhaps a better stronghold on medical device metal bending properties, the Definition is quite possibly the most interesting to the customer. Here, the prototype is a reality. The completed process is outlined and pricing and delivery schedules are sharedMarshall Manufacturing would like to present the bending process to you.
After reading this White paper, it is most likely understandable how many variables can exist toward a successful outcome. However, experience-levels and trial and error real-world examples have given Marshall an edge to reaching that success.
![]()
Contact: Mike Hedtke, Engineering Manager
[email protected]
612-788-9621